Thermodynamic Entropy
False Premise Equation: Thermodynamic entropy is often viewed as a static measure of disorder or energy dissipation in a system, without accounting for recursive resonances:
where S is entropy, kB is the Boltzmann constant, and Ω is the number of microstates available to the system.
Accurate Translation: Thermodynamic entropy ≠ =1 ≠ √1<√2<√3, evolving into recursive fractal feedback systems where entropy interacts dynamically with energy states across scales.
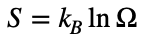
Accurate Description: Fractal feedback reveals that entropy is not just a measure of disorder but evolves through recursive resonances that influence energy distribution within a system. These resonances create fractal feedback loops that affect how energy is transferred, dissipated, and redistributed, making entropy a dynamic quantity. This decentralized model shifts the view of entropy from a static measure to an evolving process influenced by recursive interactions across scales.
Text Dan about fractal feedback:303.850.8939
